Month: January 2014
Quote of the day – Russian Humour
Russian men losing years to vodka: spirit linked to 35% of cases where a man dies before age 55, with study citing national sport of spectacular drunkenness.
http://www.theguardian.com/world/2014/jan/31/russian-men-losing-years-to-vodka
Terry: Vera, did you know that 25% of Russian men die before the age of 55?
Vera: Yes, I did, my dad was one of them.
Stalin is giving a speech when somebody sneezes. He asks, “who did that?”. Silence. He says, “kill the first row”. He asks, “who did that?”. Silence. He says, “kill the second row”. He asks, “who did that?”. An arm shakes upwards and a small voice says “me”. “Bless you”, says Stalin.
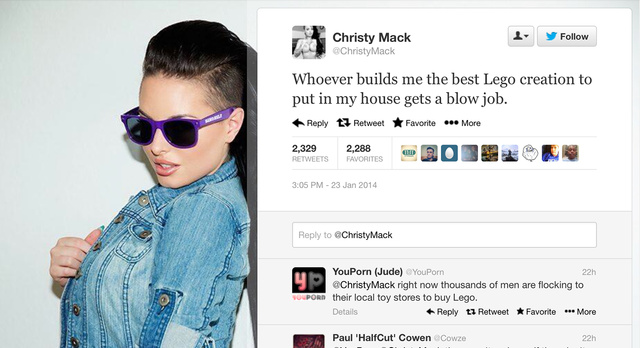
A rather unusual twitter competition
The contest:
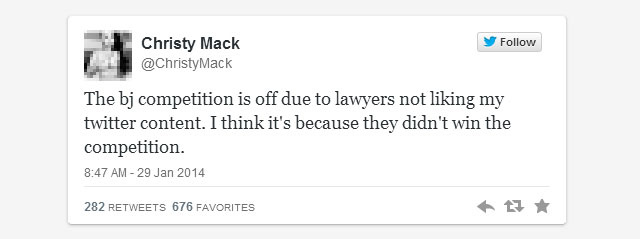
The corporate legal response:
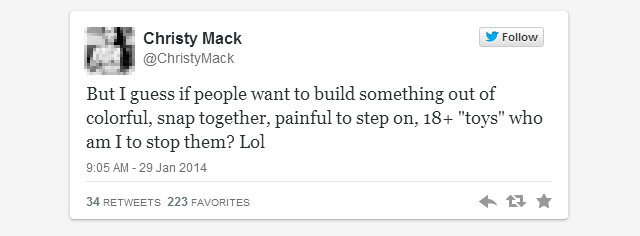
The two finger salute:
[gallery] Disney Princesses as pr0n stars

You must be logged in to be able to see this content.
Eureka!
Never a good feeling
Having to sit through a supposedly one-hour presentation, which goes over by 50%, and having your brain melt out of your ears and nose, and the only thought you have leaving the room is “well, that’s 1.5 hours of my life I’ll never get back”, is not a happy feeling.
Mom sitrep
So, according to the docs at her local hospital, she has two broken ribs and possibly a punctured lung. The ribs are broken high up on her back, which indicate a significant force of impact. She’s been given fairly hefty painkillers and she was kept under observation for her lung with more xrays planned for tomorrow.
Bit less lucky than we assumed.
My mom is under observation at the hospital. She was feeling some pain today and went for some more xrays. More info to come. It’s like exercising. It always hurts more, not the following day, but the one after that.
Just a cool picture.


Just because.
Edit: Yulia Brodskaya was born in Russia (Moscow); prior to moving to the UK in 2004 she was interested in diverse creative practices ranging from Textile Painting, Origami and Collage to more traditional Fine Art practices. Following an MA in Graphic Communication (2006, University of Hertfordshire) she continued to experiment and explore ways of bringing together all the things she likes most: typography, paper, and highly detailed hand-made craft objects. She has swiftly earned an international reputation for her innovative paper illustrations and continues to create beautifully detailed paper designs for clients all around the world.
One for all you chemistry geeks
Derek Lowe is an organic chemist. He’s worked for several major pharmaceutical companies since 1989 on drug discovery projects against schizophrenia, Alzheimer’s, diabetes, osteoporosis and other diseases. He also has a very amusing blog that contains, among other gems, a comprehensive list of things he’ll never work with.
http://pipeline.corante.com/archives/things_i_wont_work_with/
Some choice quotes:
Mercury Azides: Explosions are definitely underappreciated as a mixing technique, but in this case, they are keeping you from forming any larger crystals, a development which the paper says, with feeling, “should be avoided by all means”.
Azidoazide Azides: We’re talking high-nitrogen compounds here, and the question is not whether such things are going to be explosive hazards. (That’s been settled by their empirical formulas, which generally look like typographical errors). The question is whether you’re going to be able to get a long enough look at the material before it realizes its dream of turning into an expanding cloud of hot nitrogen gas.
Selenophenol: The chemical literature has numerous examples of people who are at a loss for words when it comes to describing its smell, but their attempts are eloquent all the same. “Imagine 6 skunks wrapped in rubber innertubes and the whole thing is set ablaze. That might approach the metaphysical stench of this material.” So we’ll start with that.
Hexanitrohexaazaisowurtzitane: There’s a recent report of a method to make a more stable form of it, by mixing it with TNT. Yes, this is an example of something that becomes less explosive as a one-to-one cocrystal with TNT. Although, as the authors point out, if you heat those crystals up the two components separate out, and you’re left with crystals of pure CL-20 soaking in liquid TNT, a situation that will heighten your awareness of the fleeting nature of life.
Dioxygen Difluoride: At seven hundred freaking degrees, fluorine starts to dissociate into monoatomic radicals, thereby losing its gentle and forgiving nature. But that’s how you get it to react with oxygen to make a product that’s worse in pretty much every way.
Chalcogen Polyazides: The experimental section of the paper enjoins the reader to wear a face shield, leather suit, and ear plugs, to work behind all sorts of blast shields, and to use Teflon and stainless steel apparatus so as to minimize shrapnel.
Chlorine Trifluoride: It is apparently about the most vigorous fluorinating agent known, and is much more difficult to handle than fluorine gas. That’s one of those statements you don’t get to hear very often. The compound also a stronger oxidizing agent than oxygen itself, which also puts it into rare territory. ”It is, of course, extremely toxic, but that’s the least of the problem. It is hypergolic with every known fuel, and so rapidly hypergolic that no ignition delay has ever been measured. It is also hypergolic with such things as cloth, wood, and test engineers, not to mention asbestos, sand, and water-with which it reacts explosively. It can be kept in some of the ordinary structural metals-steel, copper, aluminium, etc.-because of the formation of a thin film of insoluble metal fluoride which protects the bulk of the metal, just as the invisible coat of oxide on aluminium keeps it from burning up in the atmosphere. If, however, this coat is melted or scrubbed off, and has no chance to reform, the operator is confronted with the problem of coping with a metal-fluorine fire. For dealing with this situation, I have always recommended a good pair of running shoes.”


